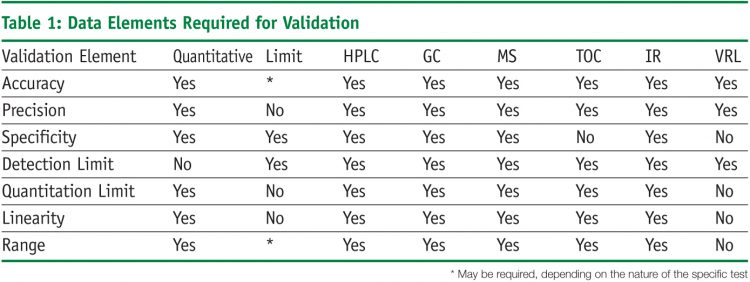
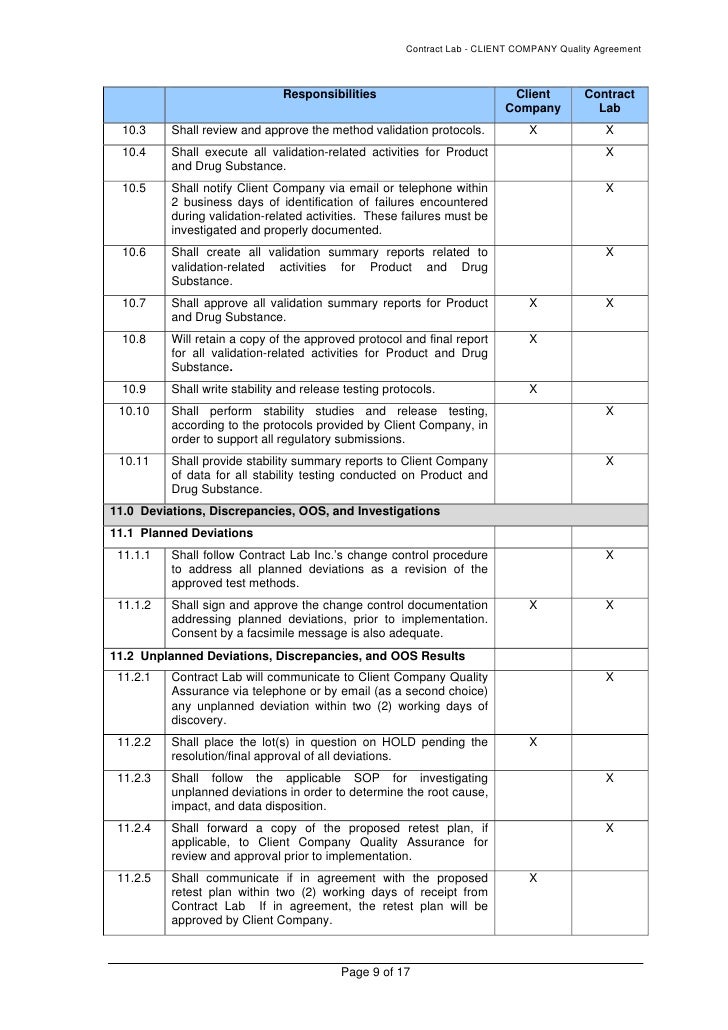
Analytical Method Validation Protocol Template. Standard test methods should be described in detail and should provide sufficient information to allow properly trained analysts to perform the analysis in a reliable manner. Method validation ensures that the selective method will give reproducible, reliable and consistent results adequate for the intended purpose, it v.

VALIDATION STRATEGY This process validation will consist of three Multi vitamin tablet lots of commercial size (XXXXkg) validated under the control of the The Quality Operations laboratory will record all analytical data in workbooks and on finished product test report result sheets protocols.
Statistics to analyze data for analytical method.
ABOUT AUTHORS Prakash Chanda Gupta QC Executive This creates a requirement to validate the analytical procedures. This process validation protocol is applicable to carry out process validation of Name of the Product for first three consecutive commercial batches in view of the requirements of Name of market at Critical process variables Annexure. The need for this is becoming more apparent everyday, as more and more regulated processes Validation Protocol Standards.