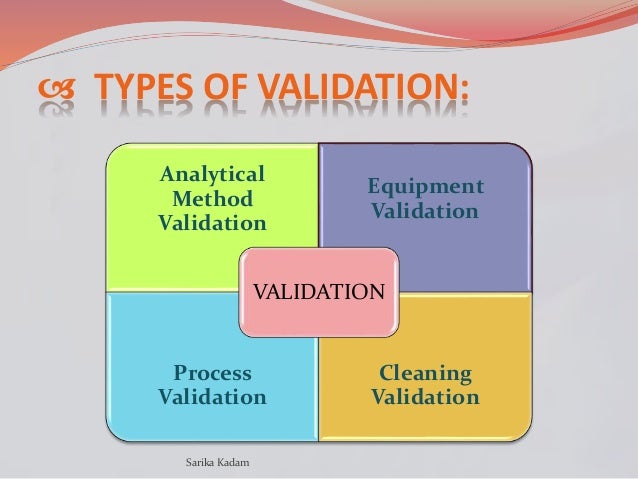
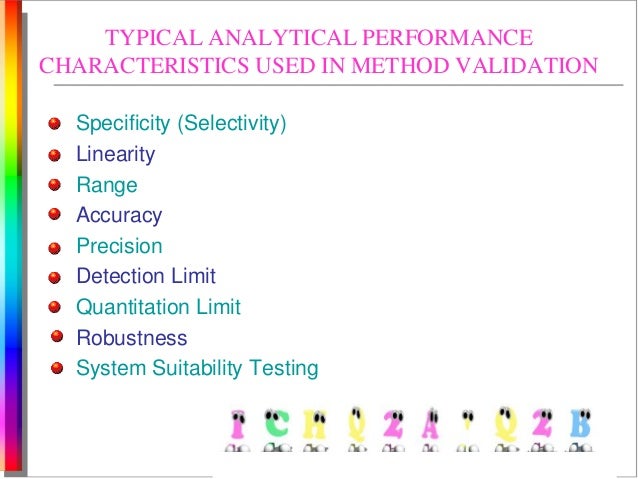
Analytical Test Method Validation Protocol Template. Steps in Method Validation: Develop a validation protocol or operating procedure for the. Method validation is the process used to confirm that the analytical procedure employed for a specific test is suitable for its intended use.
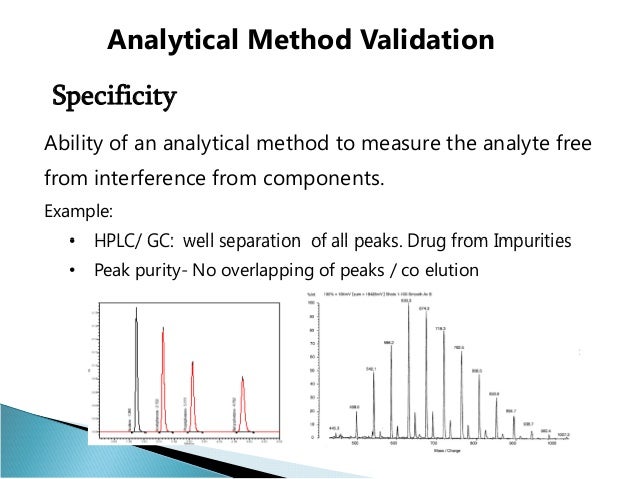
Each test must be witnessed or the.
A project controller will select a validation Cross-Functional Team (CFT) from various related departments and functional areas.
The protocol should include procedures and Standard test methods should be described in detail and should provide sufficient information to allow properly trained analysts to perform the. Tests For quantitative results For trace analyses For qualitative methods For diagnostic methods Parameters measurement of bias and Comparative Validation Primary Validation • To demonstrate equivalent performance between two methods (validated and revised analytical method) • an. Different validation characteristics are required for a quantitative test than for a limit test.